MedQdoc: Your Complete Digital eQMS for Medical Device Companies
MedQdoc is a digital Quality Management System (eQMS) designed for Medtech companies.
Do you need an efficient, compliant, and user-friendly solution to meet ISO 13485, MDR, IVDR and/or FDA requirements?
Ensuring an Effective QMS Journey for Your Medical Device Company
MedQdoc is a digital Quality Management System (eQMS) created by medical device quality and regulatory compliance experts.
It’s designed to make life easier for Medtech companies by providing a logical, compliant, and efficient workflow.
Functions and information you use daily are just a click away. Smart search tools help you quickly find what you need. Your documents, process maps, and case management modules are intelligently linked — ensuring full traceability and control. Pre-built templates are written by quality and regulatory professionals, offering a ready-to-use yet flexible system that adapts to your organization’s needs.
If you have specific requirements or questions, please don’t hesitate to get in touch.

Get up and running quickly with MedQdoc eQMS
Our experience of user integration has been extremely positive with this easy-to-use medical device quality management software. With core training in place users can quickly engage with the system and advance effectively to actual application.
Quickly start building and at the same time using your quality management system with pre-prepared QMS templates, technical documentation and other elements specifically created for the medical device industry by quality and regulatory compliance experts. You can feel confident that if you follow the structure of MedQdoc that your QMS will be compliant to ISO13485, QSR and MDR/IVDR.
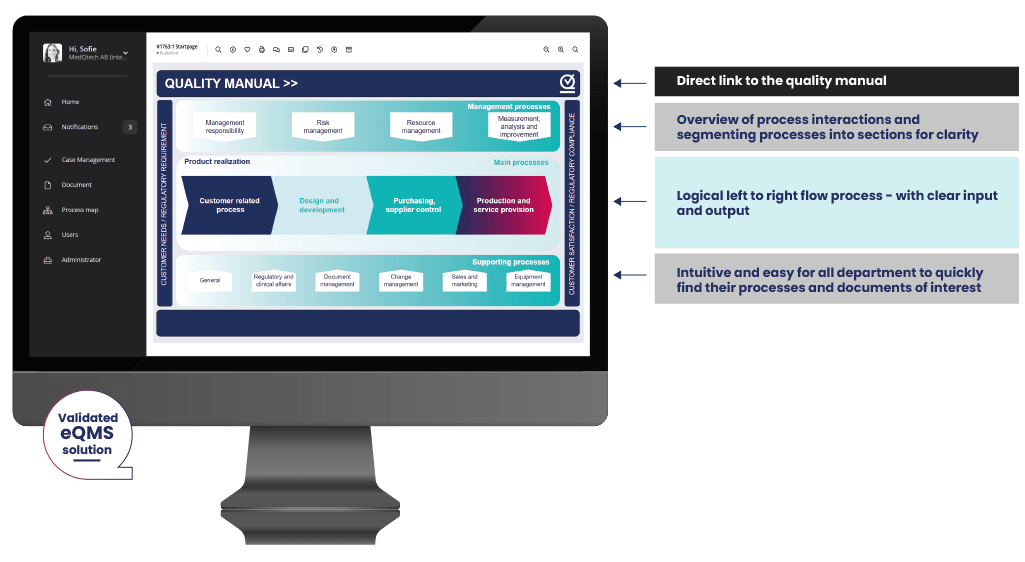
Intuitive, user friendly and simple medical device quality management software
A key subject. Your eQMS system will be used across multiple departments and employees across your business. Not all of them can be eQMS experts. MedQdoc makes it easy.
- Great start page. The intuitive start page quickly navigates you to your focus areas or documents of interest in the eQMS.
- Simple and focused. MedQdoc is focused on medical devices and the structure is set up accordingly. MedQdoc guides you to your processes and workflows, shows you only what you need, and is easily adapted to meet your business needs.</li
- Intelligent linking. To provide you with a smooth workflow, MedQdoc intelligently links documents and procedures with related process maps, documents and cases for easy navigation.
Templates, technical documentation, validation documents
– all included
Everything is included in MedQdoc and all templates are independently written by medical device quality and regulatory compliance specialists. This offers genuine value for your organisation.
A specialist medical device quality management software solution, MedQdoc contains all the document templates that you need to build up your regulatory compliant QMS, as well as technical documentation for both IVDR and MDR. It comes validated and ready to use, with all underlying validation documents included. This means that you have the opportunity later to adapt and make changes either yourself or with our support.
It is important to note that you can adapt all templates for your organization.
Simple and effective document control
A medical device quality management software built to provide effective collaboration and assured compliance.


“Can I have a list of all QMS documents and which paragraph in ISO13485 they are linked to?” Yes, of course! And you can also instantly see what paragraphs in QSR and MDR/IVDR they are connected to.
Auditors love MedQdoc
It’s a bold statement, but one we believe in. When MedQdoc is involved in an audit we get a lot of positive feedback.
Why? Our intelligent query builder creates perfect lists of everything from a product’s DHF or DMR, to all SOPs within a specific area or part of the regulation, making it easy to find the exact documentation needed. The logical format of the start page provides a real benefit during audits, along with the interlinking of documents that ensures process maps are followed effectively. MedQdoc ensures that not only is everything in place, but that it is easy to find exactly what you need. Both elements are so important to an effective medical device quality management software and can make an audit much easier.Core MedQdoc eQMS functionality examples that help you with your medical device quality and regulatory compliance









