Efficient supplier management and control with MedQdoc's predefined workflows
Efficient Supplier Management and Control with MedQdoc’s Predefined Workflows
Build and maintain a compliant and reliable supplier network with MedQdoc’s validated Supplier Management functionality. Our predefined workflows make it easy to register, evaluate, approve, and continuously monitor suppliers — ensuring compliance with MDR/IVDR, ISO 13485 and FDA QSR requirements.
With full traceability, role-based approvals, and built-in risk classification, MedQdoc enables complete control and visibility across your supply chain. Every action is logged in a validated eQMS environment — supporting your company’s audit readiness and regulatory compliance.
ISO 13485 Certified | Validated eQMS solution | 21 CFR Part 11 Compliant
Medical Device Supplier Management Functionality
The supplier management functionality in MedQdoc includes structured, ready-to-use workflows that streamline qualification, approval, and re-evaluation of suppliers. All activities are securely documented with electronic signatures and linked records such as certificates, audits, and CAPAs. Automatic audit trails and version control ensure that every supplier action remains traceable and compliant.
MMedQdoc also includes built-in templates, evaluation forms, and risk-based approval lists to help you demonstrate compliance with ISO 13485 and MDR/IVDR requirements. Developed and validated by MedQtech AB — an ISO 13485-certified company — MedQdoc provides a fully validated eQMS platform trusted by medical device manufacturers worldwide.
The medical device supplier management functionality within MedQdoc includes:
Quick access to your approved supplier list
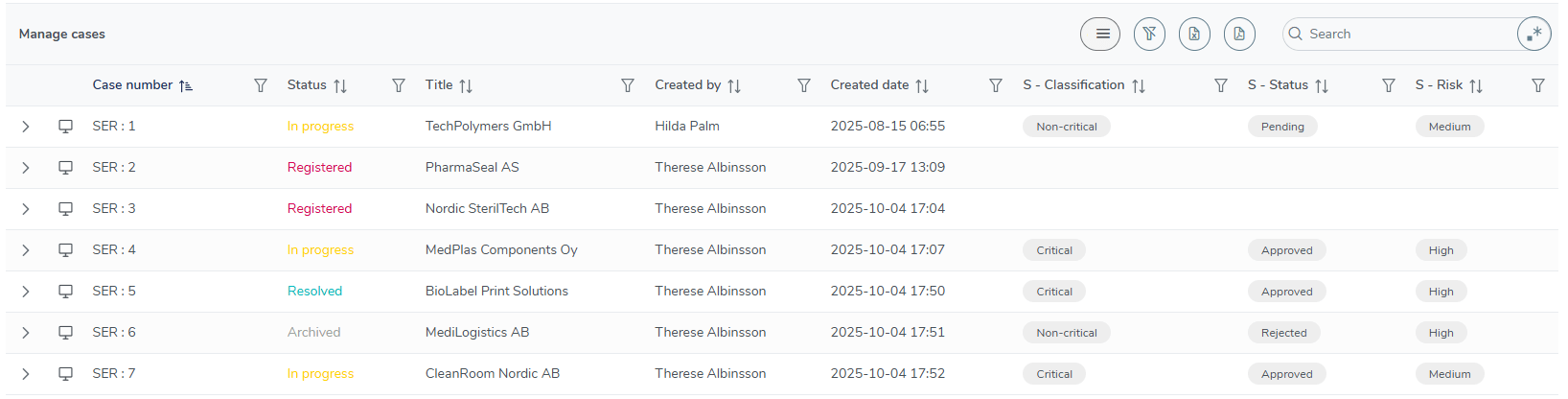
On the user-friendly MedQdoc dashboard, you’ll find your Approved Supplier List, giving you an instant overview of all suppliers — approved, pending, or rejected — along with their risk level and classification. Each supplier is managed as an individual case within MedQdoc, ensuring full traceability from registration to evaluation and re-approval. With just one click, you can filter, print, or export the list — making supplier oversight simple, transparent, and always audit-ready.
Pre-defined workflow for supplier registration
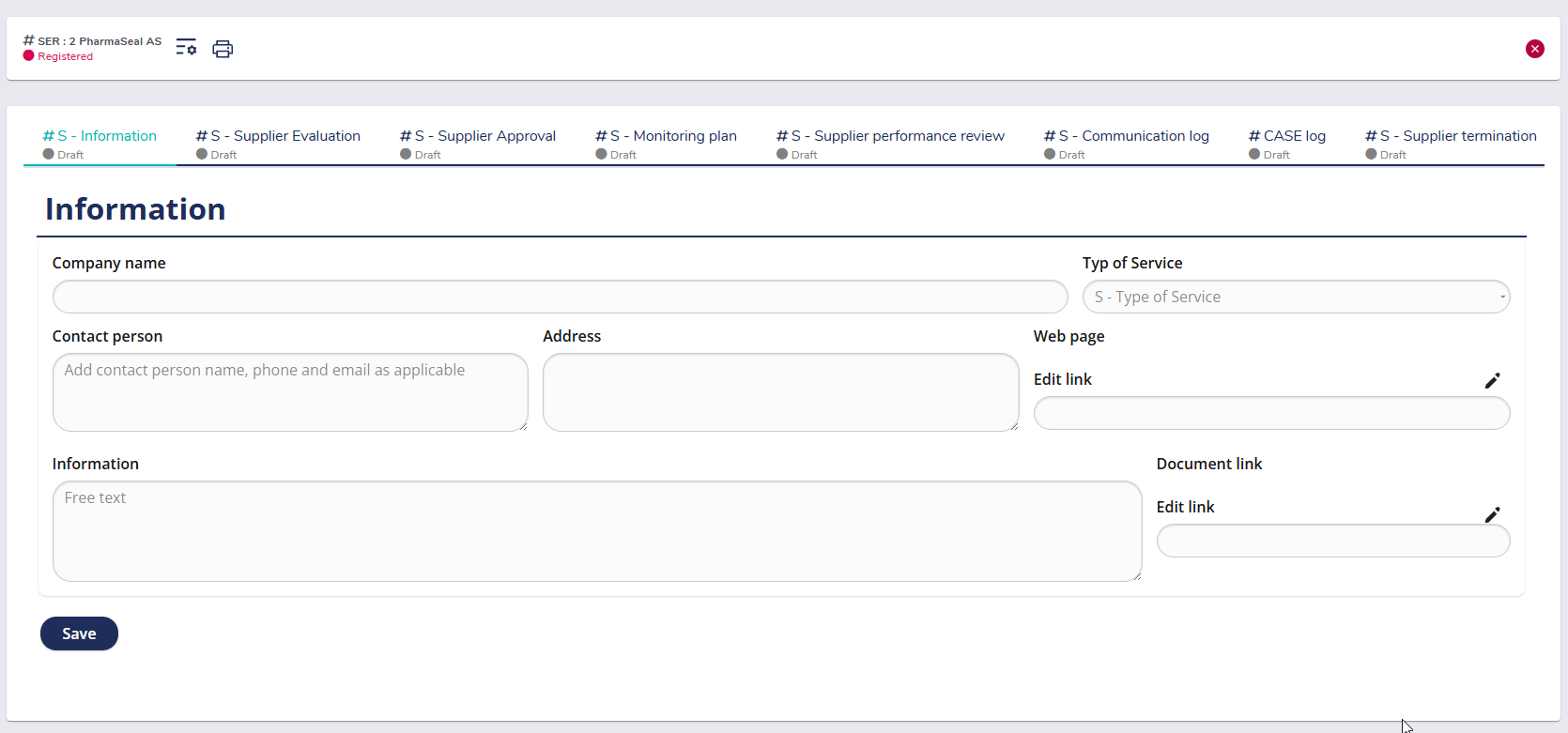
The predefined workflow in MedQdoc simplifies the way you register and classify suppliers. Each new supplier is created as an individual case, where company details, contact information, and service category are recorded in a standardized format. This ensures a consistent and compliant onboarding process aligned with ISO 13485 §7.4, providing a complete foundation for supplier evaluation and approval.
Supplier Evaluation – Structured and Risk-Based Assessment
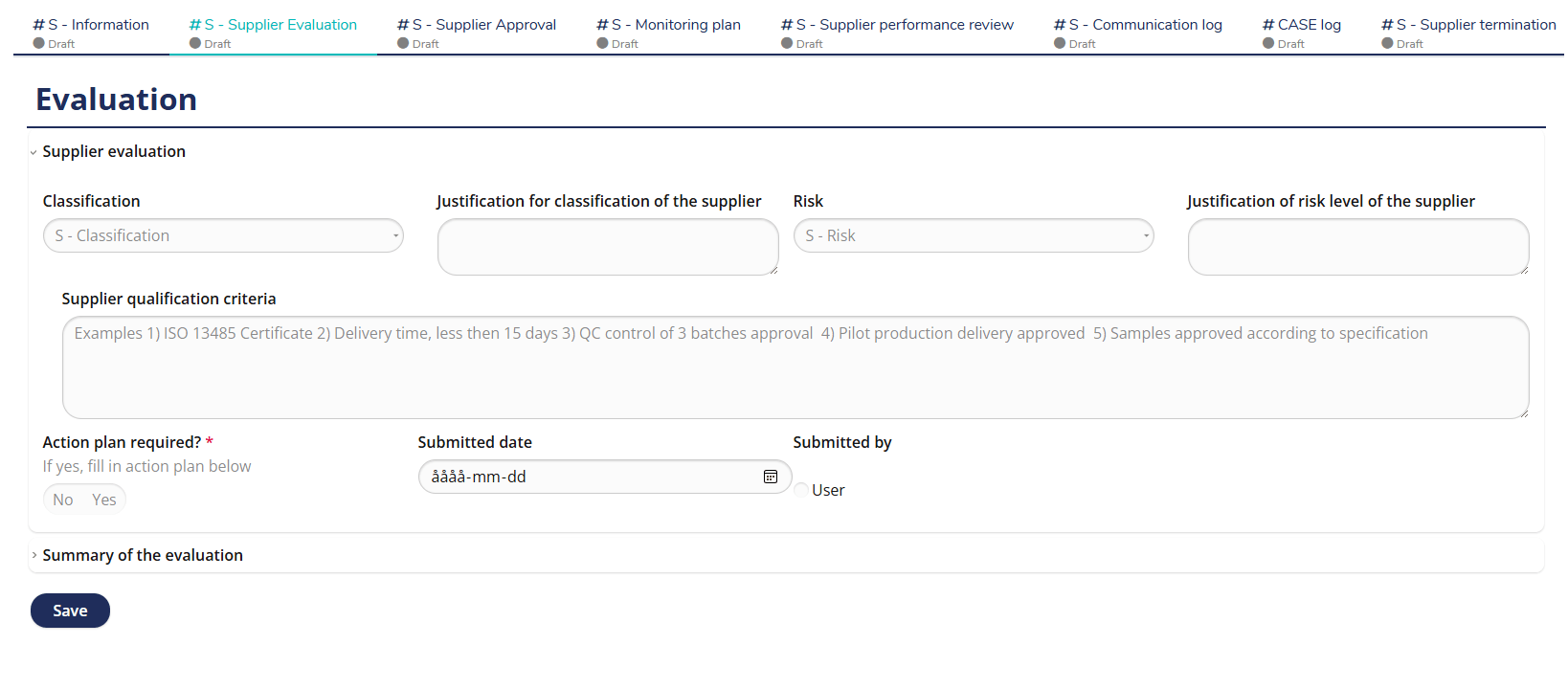
MedQdoc’s predefined Supplier Evaluation workflow helps you assess and qualify suppliers in a structured, risk-based manner. Using predefined evaluation criteria, you can document qualifications, risk levels, and justification for approval — all traceable and electronically signed. Automatic notifications remind users when evaluations are due, minimizing compliance risks and keeping your supplier records up to date. Fully aligned with ISO 13485 §7.4 and MDR supplier control requirements.
Well-structured supplier monitoring plan
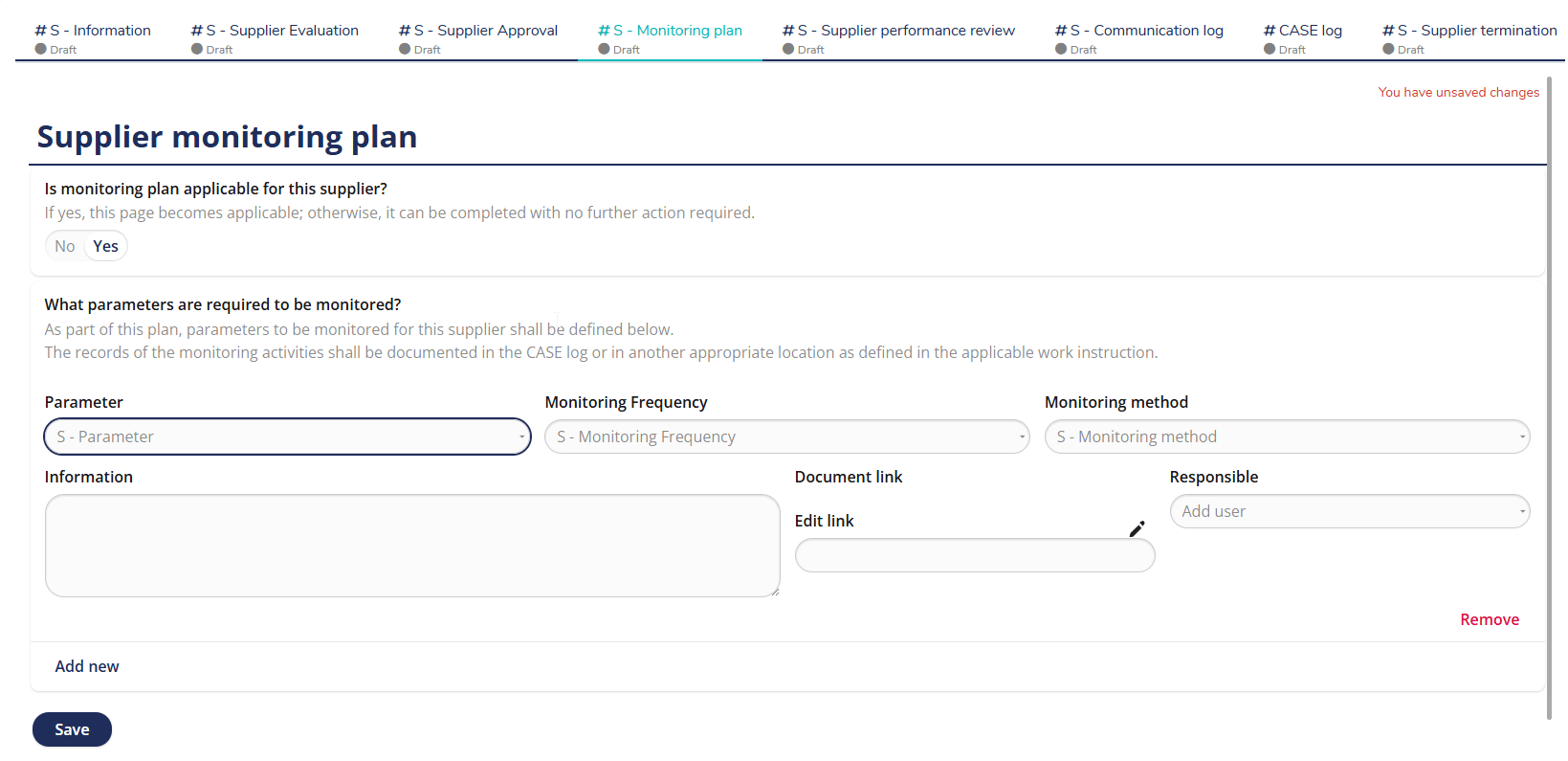
Maintain continuous oversight of supplier performance with MedQdoc’s Supplier Monitoring Plan. Define key parameters, responsible users, monitoring frequency, and methods — all in one central location. Automatic reminders help ensure supplier performance reviews are completed on time and documented according to your QMS procedures. Monitor supplier KPIs, actions, and follow-ups in a validated, audit-ready system.
Supplier Performance Review – Continuous evaluation with predefined criteria
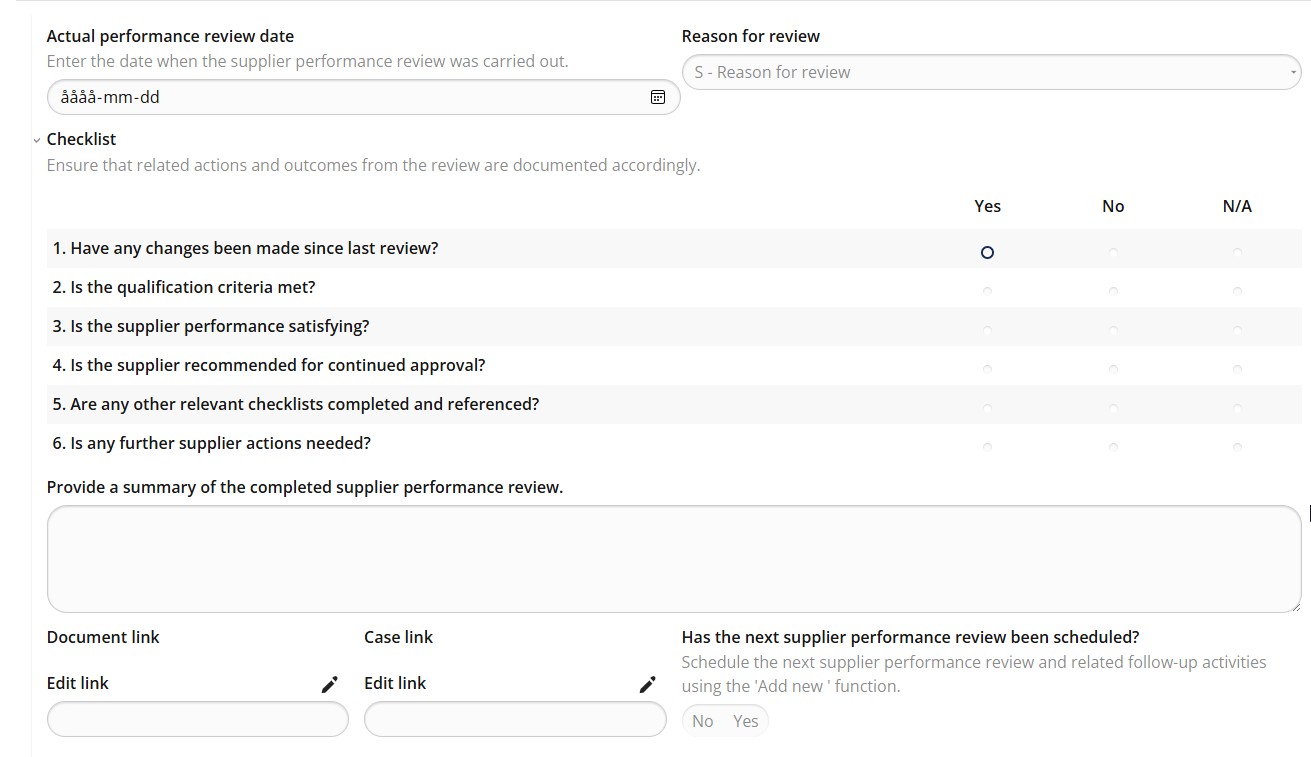
MedQdoc’s Supplier Performance Review workflow ensures that supplier evaluations are conducted consistently and on schedule. Predefined checklists guide users through every review, helping you verify supplier performance, delivery reliability, and quality results — all fully traceable within the system. Automatic notifications remind you when reviews are due, ensuring compliance with your QMS and regulatory requirements without missing critical deadlines. Certificates, agreements, and other supporting documents are securely stored and version-controlled — giving you a complete and validated supplier record from day one. MedQdoc’s document lifecycle then guides you through review and approval, ensuring full traceability and compliance throughout your supplier management process.
Supplier Questionnaire – Secure and Simple Supplier Information Collection
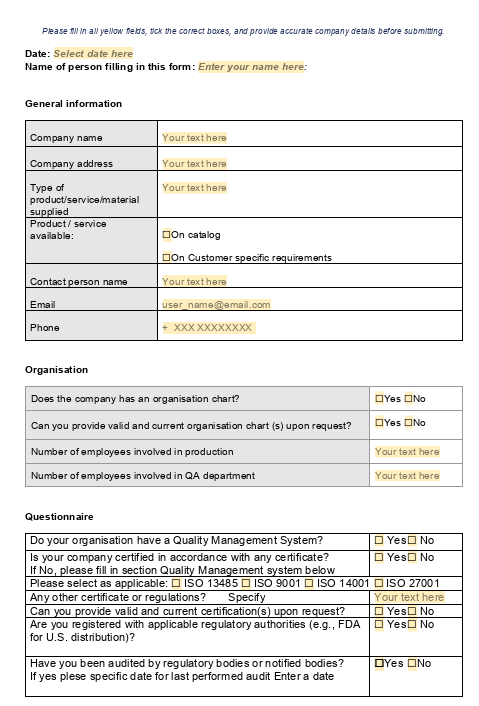
Simplify your supplier onboarding process with MedQdoc’s Supplier Questionnaire. Send a digital form to your suppliers and easily upload their completed information into MedQdoc, automatically linking it to the relevant supplier case. MedQdoc’s document lifecycle then guides you through review and approval, ensuring full traceability and compliance throughout your supplier management process.
MedQdoc medical device supplier management helps you to comply effectively with:
MDR / IVDR
ISO
13485
ISO
14971
QSR
(FDA)
CE-marking
process
21 CFR
Part 11
![]()
Designed by medical device quality and regulatory compliance experts.
![]()
Includes over 160 QMS and MDR/IVDR technical documentation templates for medical device compliance.
![]()
Intuitive and user friendly; simple and effective ISO 13485 document control.
![]()
MedQdoc is loved by auditors.
“I would absolutely recommend MedQdoc to other companies in the medical device world. Without a doubt, using MedQdoc has been a key factor in the success of our ISO 13485 certification process.”
Kristian Nisja, QA & HSE Manager,
NORBIT EMS Selbu

MedQdoc has extensive functionality in all of the QMS areas below, please talk to the MedQdoc team for specific queries that you may have:
Quality Management System
Change Management
Supplier Management
Training Management
CAPA
Non- Conformities
Complaint Handling
Customized Case Flows
Template Management
Technical Documentation



